https://www.npr.org/sections/health-shots/2022/04/28/ [login to see] /moderna-asks-fda-to-authorize-first-covid-19-vaccine-for-very-young-children
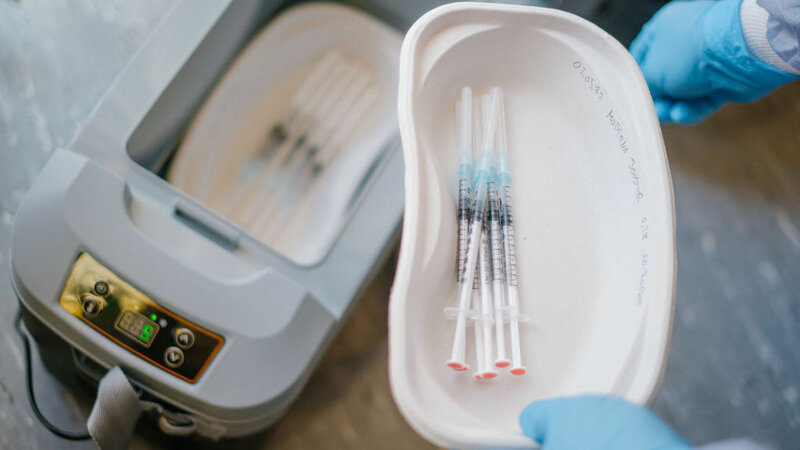
Moderna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
In a study involving about 6,700 children, the company said two-doses of the vaccine administered 28 days apart to children ages 6 months to less than 6 years triggered levels of antibodies equivalent to what has protected older children and adults.
"We are proud to share that we have submitted for authorization for our COVID-19 vaccine for young children," said Stéphane Bancel, Moderna's chief executive officer, in a statement. "We believe [the vaccine] will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19, and will be especially welcomed by parents and caregivers."
The vaccine appears to be about 51 percent effective for children ages 6 months to less than 2 years, and 37 percent effective for those ages 2 to less than 6 years, the company says.
"That means that you're going to reduce your chances of getting disease by about a half. That's very important for these kids," Dr. Paul Burton, Moderna's chief medical officer, told NPR in an interview.

 Children
Children Medical
Medical Health
Health Drugs
Drugs Pharmaceuticals
Pharmaceuticals