Posted on Jun 22, 2022
Kids under 5 receive first Covid-19 doses in Seattle
2.7K
8
3
5
5
0
Posted >1 y ago
Responses: 3
Thank you my friend and brother-in-Christ PO1 William "Chip" Nagel for making us aware
that children under 5 are receiving their first of three cumulative does of COVID vaccine in Seatttle Washington.
The Serious Concerns The FDA Raised While Still Voting Yes To Jab Your Toddlers – Moderna
theRealDTrain Published June 20, 2022 22 Views
Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age"
https://rumble.com/v196au7-the-serious-concerns-the-fda-raised-while-still-voting-yes-to-jab-your-todd.html
Images;
1. June 15, 2022, three-year-old Andel Good was the first youngster to receive the Pfizer-BioNTech Covid-19 vaccine at Stanford Medicine. His parents, Otavio and Zina Good, enrolled him in a clinical trial for children aged 6 months through 4 years.
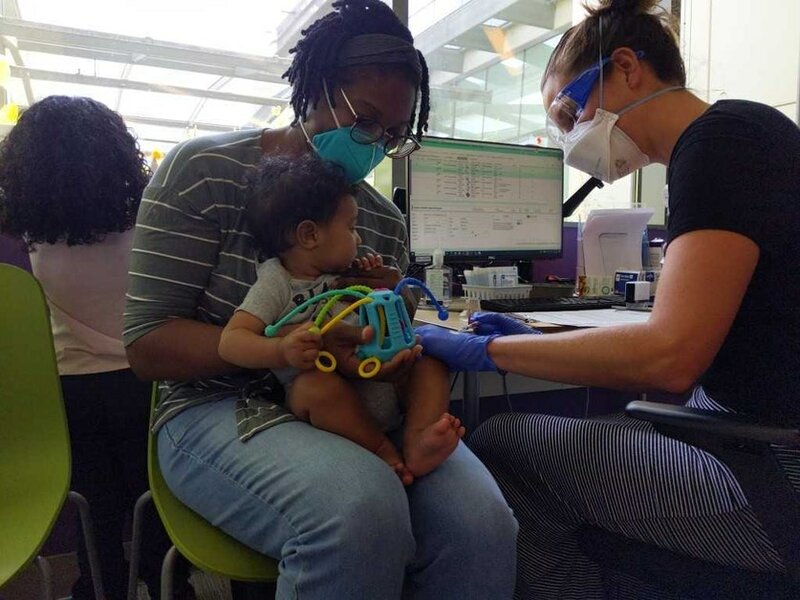
2. Sandino, eight months old, gets his first shot of the Covid-19 vaccine at Seattle Children's on June 21, 2022.
3. European girl receiving COVID vaccine
4. Toddler receiving a COVID vaccination
5. Needles containing the newly approved Covid-19 vaccine for children between the ages of 6 months and 5 years sit in a container at Children's Hospital on June 21
BLUF "Paul Offit, MD, of the Children's Hospital of Philadelphia, said he was surprised that there was "no protection" after the second dose, noting the importance of communicating to families that their child may have to wait a few months before the vaccine is effective. "I do worry that parents aren't necessarily going to know that after two doses, they may not be protected at all," he said.'
Background from {[medpagetoday.com/infectiousdisease/covid19vaccine/99270]}
by Amanda D'Ambrosio, Enterprise & Investigative Writer, MedPage Today June 15, 2022
An advisory committee recommended that the FDA give emergency use authorization (EUA) to both Moderna and Pfizer/BioNTech's mRNA COVID-19 vaccines for infants, toddlers, and preschool kids.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted 21-0 in favor of expanded access to each vaccine, in another unanimous decision that the benefits of the vaccines outweigh the risks in these young kids.
Moderna is seeking authorization for two 25-mcg doses administered 3 weeks apart for kids ages 6 months to 5 years, while Pfizer's request is for three 3-mcg doses for kids ages 6 months to 4 years, which would be administered 3 weeks apart between the first and second shots, and 8 weeks later for the third.
Several members of the advisory committee agreed on the importance of giving families the choice to vaccinate their kids under 5 -- a group still waiting for access to the vaccine.
"I know that there are a lot of very relieved parents, almost certainly who are listening to this right now," said panelist Jay Portnoy, MD, of Children's Mercy Hospital in Kansas City. "They've been waiting for a very long time."
However, the panelists were split on the risks from COVID-19 in this age group. While some stated that many kids who become infected get mild illness, others noted the severe outcomes that children have experienced compared with other disease outbreaks.
During introductions, Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, said there was a "troubling" rate of hospitalizations among young kids during the Omicron surge -- worse than what we typically see in a terrible influenza season. As of May 28, there were 442 COVID-related deaths in kids under 4, according to the CDC.
"I think we have to be careful that we don't become numb to the number of pediatric deaths because of the overwhelming number of older deaths here," Marks said. "Every life is important."
Hayley Altman-Gans, MD, a panelist from Stanford University Medical Center, said that the committee need not underplay the severity of COVID as a pediatric disease. "Prevention is really the way to go," she noted.
Some members voiced concerns about the efficacy of the Pfizer vaccine, specifically after the first two doses. Paul Offit, MD, of the Children's Hospital of Philadelphia, said he was surprised that there was "no protection" after the second dose, noting the importance of communicating to families that their child may have to wait a few months before the vaccine is effective.
"I do worry that parents aren't necessarily going to know that after two doses, they may not be protected at all," he said.
Data on Moderna's two-dose COVID-19 vaccine, released in FDA briefing documents earlier this week, came from mRNA-1273-P204, an ongoing phase II/III clinical trial that included 4,000 kids ages 2 to 5 (3,000 of whom received the vaccine and 1,000 who received a placebo), as well as 2,350 kids ages 6 to 23 months (1,761 who received the vaccine and 589 who received a placebo). The median duration of follow-up was about 2 months for both age groups.
The Moderna vaccine met immunobridging success criteria in both groups. Neutralizing antibody geometric mean titers and seroresponse rates were noninferior to those seen in adults ages 18 to 25.
Preliminary vaccine efficacy was 36.8% (95% CI 12.5-54.0) for those ages 2 to 5, and 50.6% (95% CI 21.4-68.6) for those ages 6 to 23 months. Efficacy was evaluated during a period when the Omicron variant was predominant.
Adverse events (AEs) were mild to moderate in severity. Fevers were more common in the vaccine group than the placebo group, and occurred more frequently after the second dose. Rates of fever among the youngest age group were 21-26%, with a high fever (over 104°F) reported in less than 0.4% of children in this cohort.
A separate FDA briefing document included data on the efficacy of Pfizer/BioNTech's three-dose COVID vaccination series among children ages 6 months to 4 years. Study C4591007, an ongoing phase II/III trial, included 1,835 vaccine recipients and 915 placebo recipients ages 2 to 4 years, and 1,178 vaccine recipients and 598 placebo recipients ages 6 to 23 months.
The Pfizer vaccine also met immunobridging success criteria for both age groups. Neutralizing antibody geometric mean titers and seroresponse rates were noninferior to those seen in young adults ages 16 to 25.
Vaccine efficacy among both groups combined was 80.4% (95% CI 14.1-96.7), and was 75.6% (95% CI -369.1 to 99.6) for those ages 6 to 23 months, and 82.4% (95% CI -7.6 to 98.3) for those ages 2 to 4 years.
Overall, three COVID-19 cases were reported in participants who received the vaccine versus seven in the placebo group.
Common adverse reactions among infants and toddlers included irritability, drowsiness, decreased appetite, and tenderness at the injection site. Among kids ages 2 to 4, common side effects were pain and redness at the injection site and fatigue.
There were no reported cases of myocarditis or pericarditis for either vaccine.
Panelists noted that regulators will continue to monitor the safety and efficacy of the vaccines, taking future surveillance seriously as shots are administered to a larger population. However, they were clear that giving families a choice to vaccinate their little ones will benefit many.
"The lack of the vaccines for these young children has been a gap for many, and has really had an impact on their lives, so I think this was really very positive," said Jeannette Yen Lee, PhD, of the University of Arkansas for Medical Sciences.
"I do think it's clear that the story isn't over," she added. "The pandemic has taken some different twists and turns, and there may be more options for these children in the future."
FYI MAJ Byron Oyler MAJ Dale E. Wilson, Ph.D. PO1 Jeff ChandlerCMDCM John F. "Doc" Bradshaw SGT Denny Espinosa Kim Bolen RN CCM ACM Sgt (Join to see) CSM Chuck Stafford SFC Bernard Walko SGT James MurphyMaj Bill Smith, Ph.D. MAJ Dale E. Wilson, Ph.D. LTC Kevin CoughlinCPO John Bjorge 2LT Jeff Fortin SSG Everett Wilson SFC Chuck Martinez 1LT Vance Titus
that children under 5 are receiving their first of three cumulative does of COVID vaccine in Seatttle Washington.
The Serious Concerns The FDA Raised While Still Voting Yes To Jab Your Toddlers – Moderna
theRealDTrain Published June 20, 2022 22 Views
Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age"
https://rumble.com/v196au7-the-serious-concerns-the-fda-raised-while-still-voting-yes-to-jab-your-todd.html
Images;
1. June 15, 2022, three-year-old Andel Good was the first youngster to receive the Pfizer-BioNTech Covid-19 vaccine at Stanford Medicine. His parents, Otavio and Zina Good, enrolled him in a clinical trial for children aged 6 months through 4 years.
2. Sandino, eight months old, gets his first shot of the Covid-19 vaccine at Seattle Children's on June 21, 2022.
3. European girl receiving COVID vaccine
4. Toddler receiving a COVID vaccination
5. Needles containing the newly approved Covid-19 vaccine for children between the ages of 6 months and 5 years sit in a container at Children's Hospital on June 21
BLUF "Paul Offit, MD, of the Children's Hospital of Philadelphia, said he was surprised that there was "no protection" after the second dose, noting the importance of communicating to families that their child may have to wait a few months before the vaccine is effective. "I do worry that parents aren't necessarily going to know that after two doses, they may not be protected at all," he said.'
Background from {[medpagetoday.com/infectiousdisease/covid19vaccine/99270]}
by Amanda D'Ambrosio, Enterprise & Investigative Writer, MedPage Today June 15, 2022
An advisory committee recommended that the FDA give emergency use authorization (EUA) to both Moderna and Pfizer/BioNTech's mRNA COVID-19 vaccines for infants, toddlers, and preschool kids.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted 21-0 in favor of expanded access to each vaccine, in another unanimous decision that the benefits of the vaccines outweigh the risks in these young kids.
Moderna is seeking authorization for two 25-mcg doses administered 3 weeks apart for kids ages 6 months to 5 years, while Pfizer's request is for three 3-mcg doses for kids ages 6 months to 4 years, which would be administered 3 weeks apart between the first and second shots, and 8 weeks later for the third.
Several members of the advisory committee agreed on the importance of giving families the choice to vaccinate their kids under 5 -- a group still waiting for access to the vaccine.
"I know that there are a lot of very relieved parents, almost certainly who are listening to this right now," said panelist Jay Portnoy, MD, of Children's Mercy Hospital in Kansas City. "They've been waiting for a very long time."
However, the panelists were split on the risks from COVID-19 in this age group. While some stated that many kids who become infected get mild illness, others noted the severe outcomes that children have experienced compared with other disease outbreaks.
During introductions, Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, said there was a "troubling" rate of hospitalizations among young kids during the Omicron surge -- worse than what we typically see in a terrible influenza season. As of May 28, there were 442 COVID-related deaths in kids under 4, according to the CDC.
"I think we have to be careful that we don't become numb to the number of pediatric deaths because of the overwhelming number of older deaths here," Marks said. "Every life is important."
Hayley Altman-Gans, MD, a panelist from Stanford University Medical Center, said that the committee need not underplay the severity of COVID as a pediatric disease. "Prevention is really the way to go," she noted.
Some members voiced concerns about the efficacy of the Pfizer vaccine, specifically after the first two doses. Paul Offit, MD, of the Children's Hospital of Philadelphia, said he was surprised that there was "no protection" after the second dose, noting the importance of communicating to families that their child may have to wait a few months before the vaccine is effective.
"I do worry that parents aren't necessarily going to know that after two doses, they may not be protected at all," he said.
Data on Moderna's two-dose COVID-19 vaccine, released in FDA briefing documents earlier this week, came from mRNA-1273-P204, an ongoing phase II/III clinical trial that included 4,000 kids ages 2 to 5 (3,000 of whom received the vaccine and 1,000 who received a placebo), as well as 2,350 kids ages 6 to 23 months (1,761 who received the vaccine and 589 who received a placebo). The median duration of follow-up was about 2 months for both age groups.
The Moderna vaccine met immunobridging success criteria in both groups. Neutralizing antibody geometric mean titers and seroresponse rates were noninferior to those seen in adults ages 18 to 25.
Preliminary vaccine efficacy was 36.8% (95% CI 12.5-54.0) for those ages 2 to 5, and 50.6% (95% CI 21.4-68.6) for those ages 6 to 23 months. Efficacy was evaluated during a period when the Omicron variant was predominant.
Adverse events (AEs) were mild to moderate in severity. Fevers were more common in the vaccine group than the placebo group, and occurred more frequently after the second dose. Rates of fever among the youngest age group were 21-26%, with a high fever (over 104°F) reported in less than 0.4% of children in this cohort.
A separate FDA briefing document included data on the efficacy of Pfizer/BioNTech's three-dose COVID vaccination series among children ages 6 months to 4 years. Study C4591007, an ongoing phase II/III trial, included 1,835 vaccine recipients and 915 placebo recipients ages 2 to 4 years, and 1,178 vaccine recipients and 598 placebo recipients ages 6 to 23 months.
The Pfizer vaccine also met immunobridging success criteria for both age groups. Neutralizing antibody geometric mean titers and seroresponse rates were noninferior to those seen in young adults ages 16 to 25.
Vaccine efficacy among both groups combined was 80.4% (95% CI 14.1-96.7), and was 75.6% (95% CI -369.1 to 99.6) for those ages 6 to 23 months, and 82.4% (95% CI -7.6 to 98.3) for those ages 2 to 4 years.
Overall, three COVID-19 cases were reported in participants who received the vaccine versus seven in the placebo group.
Common adverse reactions among infants and toddlers included irritability, drowsiness, decreased appetite, and tenderness at the injection site. Among kids ages 2 to 4, common side effects were pain and redness at the injection site and fatigue.
There were no reported cases of myocarditis or pericarditis for either vaccine.
Panelists noted that regulators will continue to monitor the safety and efficacy of the vaccines, taking future surveillance seriously as shots are administered to a larger population. However, they were clear that giving families a choice to vaccinate their little ones will benefit many.
"The lack of the vaccines for these young children has been a gap for many, and has really had an impact on their lives, so I think this was really very positive," said Jeannette Yen Lee, PhD, of the University of Arkansas for Medical Sciences.
"I do think it's clear that the story isn't over," she added. "The pandemic has taken some different twists and turns, and there may be more options for these children in the future."
FYI MAJ Byron Oyler MAJ Dale E. Wilson, Ph.D. PO1 Jeff ChandlerCMDCM John F. "Doc" Bradshaw SGT Denny Espinosa Kim Bolen RN CCM ACM Sgt (Join to see) CSM Chuck Stafford SFC Bernard Walko SGT James MurphyMaj Bill Smith, Ph.D. MAJ Dale E. Wilson, Ph.D. LTC Kevin CoughlinCPO John Bjorge 2LT Jeff Fortin SSG Everett Wilson SFC Chuck Martinez 1LT Vance Titus

The Serious Concerns The FDA Raised While Still Voting Yes To Jab Your Toddlers – Moderna
Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age https://web.archive.org/web/20220617125738/https://www.fda.gov/news-eve
(1)
(0)
If parents feel this is warranted, then they should go for it. I'm not sure the metrics bear out the necessity just yet
(1)
(0)
Read This Next


 Children
Children Health
Health Pharmaceuticals
Pharmaceuticals Seattle
Seattle Medical
Medical



